Background
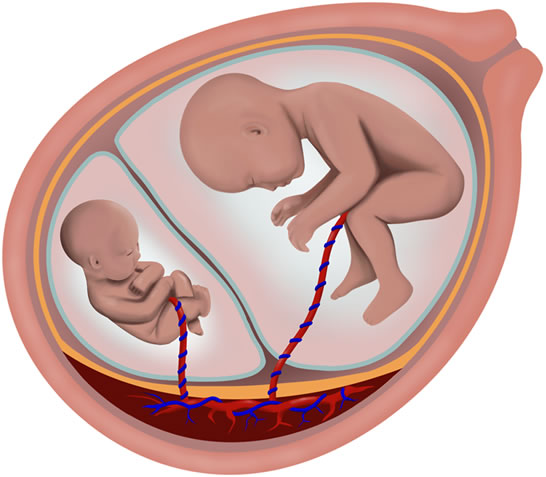 Although most pregnancies with monochorionic twins (twins that share a common placenta) are uncomplicated, the presence of a common placenta does pose a relatively increased risk to the welfare of the fetuses. The single placenta contains blood vessels that link the blood flow between the twins. Unbalanced flow of blood from one twin to the other twin may lead to a cascade of events that result in twin-twin transfusion syndrome (further information regarding this syndrome is detailed in the section titled twin-twin transfusion syndrome listed in the previous menu). Another potential problem that may occur in monochorionic twins is the disproportionate distribution of placental mass between the twins. This factor may result in poor nourishment of one of the twins, resulting in subsequent poor overall fetal growth. Because this problem typically affects only one of the fetuses, this condition has been coined selective intrauterine growth restriction (SIUGR). SIUGR is estimated to occur in approximately 10% of monochorionic twin pregnancies.
Although most pregnancies with monochorionic twins (twins that share a common placenta) are uncomplicated, the presence of a common placenta does pose a relatively increased risk to the welfare of the fetuses. The single placenta contains blood vessels that link the blood flow between the twins. Unbalanced flow of blood from one twin to the other twin may lead to a cascade of events that result in twin-twin transfusion syndrome (further information regarding this syndrome is detailed in the section titled twin-twin transfusion syndrome listed in the previous menu). Another potential problem that may occur in monochorionic twins is the disproportionate distribution of placental mass between the twins. This factor may result in poor nourishment of one of the twins, resulting in subsequent poor overall fetal growth. Because this problem typically affects only one of the fetuses, this condition has been coined selective intrauterine growth restriction (SIUGR). SIUGR is estimated to occur in approximately 10% of monochorionic twin pregnancies.
Severe cases of monochorionic twins with SIUGR show ultrasound evidence of abnormal blood flow through the umbilical artery of the poorly grown twin. In this circumstance, spontaneous death of this baby within the womb may occur in up to 40% of cases. Because of the blood vessels that link the twin’s circulatory system together, death of one twin may result in severe drop in blood pressure of the other twin and subsequent brain damage or death (up to 30%). This complication results from the hemorrhage of blood from the appropriately grown twin into the demised SIUGR twin.
Because the adverse effects to the appropriately grown twin is mediated through the blood vessels that link the circulations of the twins, it has been suggested that obliteration of these vascular communications may result in improved outcomes for the normally grown twin. Separation of the circulations me be done using the surgical techniques which were originally developed for the treatment of twin-twin transfusion syndrome.
Diagnosis
The in utero diagnosis of SIUGR is established by ultrasound. First, the presence of a monochorionic twin gestation should be confirmed. Usually ultrasounds performed earlier in the pregnancy may be useful in establishing the chorionicity (number of placentas). Ultrasound findings such as a single placenta, same fetal sex, and a “T-sign” in which the dividing membrane inserts perpendicular to the placenta are helpful in diagnosing a monochorionic twin gestation.
Once a monochorionic placentation has been established, the diagnosis of SIUGR requires the presence of two important ultrasound findings:
- The estimated fetal weight (EFW) of one twin measures less than the 10th percentile for the assigned gestational age. The EFW is calculated by measuring standard fetal biometric components via ultrasound. Because prior studies have shown negligible difference between growth curves for singleton and twin gestations in the second trimester, standards as established by Hadlock (1991) for singletons are used to assign the growth percentile.
- Persistent absent or reversed flow in the umbilical artery of the growth-restricted twin.
Finally, the diagnosis of twin-twin transfusion syndrome (TTTS) must be excluded. TTTS is diagnosed by assessing the discordance of amniotic fluid volume on either side of the dividing fetal membranes; the maximum vertical pocket (MVP) of amniotic fluid volume must be greater than or equal to 8.0 centimeters in the recipient’s sac, and less than or equal to 2.0 centimeters in the donor’s sac to secure the diagnosis of TTTS.
The findings of monochorionic diamniotic twins with SIUGR and absent or reversed end-diastolic flow in the umbilical arteries has been classified by some as SIUGR, Type II. These patients are candidates for the management options listed below. Please note that laser surgery and cord occlusion are not recommended for SIUGR, Type I (normal umbilical artery Doppler waveform), and SIUGR, Type III (intermittent absent end-diastolic flow in the umbilical artery).
Management Options and Outcomes
The treatment options along with expected pregnancy outcomes are listed below:
- Expectant Management: Prior to the development of the laser therapy outlined below, the treatment of this condition has been traditionally one of expectant management. This entails at least weekly ultrasound assessments of fetal wellbeing, amniotic fluid volume assessment, and Doppler studies of the umbilical artery, as well as sonograms to assess fetal growth about every three weeks. After 24 weeks’ gestation, parents traditionally discuss with their physicians whether there is a need for increased fetal surveillance, such as fetal heart rate monitoring, and if a course of steroids is required for fetal maturation therapy. Early delivery may be decided if fetal status is deemed nonreassuring based on fetal heart rate monitoring or ultrasound parameters. The challenge that this condition presents to parents and physicians alike is in regards to the timing of delivery. On the one hand, delay of delivery will reduce the complications associated with premature birth. On the other hand, prolongation of the pregnancy in this setting, particularly if findings suggestive of a nonreassuring fetal status are present, may result in the demise of one twin in the womb. This may occur in up to 40% of monochorionic twins with SIUGR. As described above, the death of one twin while in the womb may result in the concomitant demise of the other twin in as high as 40% of cases. If the other twin does survive, there is an increased risk of subsequent neurologic handicap. The demise of a twin results in these adverse effects on the other twin because of the blood vessels on the surface of the placenta that connect the circulatory systems of the babies – essentially linking the livelihoods of each baby to one another.
- Laser Therapy: This surgical approach utilizes an operative fetoscope to deliver laser energy that then seals off the offending blood vessels on the surface of the common placenta. Because the vascular connections between the two fetuses are sealed, no further blood exchange between the fetuses takes place. It has been theorized that elimination of the vascular communications may decrease or prevent harm to the surviving twin in the case of the demise of one twin. The magnitude of this potential benefit is unknown. A preliminary study comparing the outcomes of patients followed with expectant management versus those that underwent laser therapy did not show a difference in survival or in complications of the babies. However, this study was small and did not involve patients equally.
- Umbilical Cord Occlusion: This procedure utilizes an operative fetoscope to interrupt the flow of blood through the umbilical cord of one of the fetuses. This fetus dies and remains inside the uterus for the duration of the pregnancy. We do not offer this procedure for this condition.
- Interruption of the Pregnancy: Pregnancy termination may be chosen as an option up to 24 weeks gestation in California. We do not offer this procedure.
Candidacy for Treatment
To qualify for laster surgery, the following conditions must be met:
Inclusion Criteria
- Gestational age 16-26 weeks
- Sonographic evidence of monochorionicity
- Diagnosis of IUGR present in one twin (fetal weight at or below the 10th percentile for gestational age (Hadlock et al 1991))
In Utero Fetal Weight Standards at Ultrasound Percentiles (g) Menstrual Week 3rd 10th 50th 90th 97th 16 110 121 146 171 183 17 136 150 181 212 226 18 167 185 223 261 279 19 205 227 273 319 341 20 248 275 331 387 414 21 299 331 399 467 499 22 359 398 478 559 598 23 426 471 568 665 710 24 503 556 670 784 838 25 589 652 758 918 981 26 685 758 913 1,068 1,141 - Absent or reverse-end diastolic flow in the umbilical artery in the SIUGR twin
Exclusion Criteria
- Presence of twin-twin transfusion syndrome defined as a maximum vertical pocket (MVP) of ≤2 cm in one sac and MVP of ≥8 cm in the other sac
- Presence of major congenital anomalies (anencephaly, acardia, spina bifida) or intracranial findings in either twin: IVH, porencephalic cysts, ventriculomegaly or other findings suggestive of brain damage
- Unbalanced chromosomal complement
- Ruptured or detached membranes
- Placental Abruption
- Chorioamnionitis
- Triplets
- Refusal to be randomized or to participate in the study
- Otherwise eligible, but not able to make financial arrangements.
Details of Procedure and Outline of Care During Pregnancy
A. Expectant Management
Patients choosing to undergo expectant management will be advised to undergo weekly ultrasound examinations including Doppler studies of the umbilical artery and amniotic fluid volume. Fetal growth will be assessed every 2-4 weeks. After 24 weeks, patients may undergo frequent ultrasound examinations or fetal heart rate monitoring to assess fetal well-being. At approximately 26 weeks, steroids may be administered for enhancement of fetal lung maturity. Early delivery may be decided by the respective obstetricians if either ultrasound or fetal heart rate monitoring assessments are not reassuring of fetal well being. Of note, if the ultrasound findings consistent with twin-twin transfusion syndrome (TTTS) develop prior to 26 weeks’ gestation, then laser therapy as outlined in the TTTS section of this web site will be offered.
B. Laser Therapy
Patients choosing to proceed with laser therapy will be treated via selective laser photocoagulation of the communicating vessels. After appropriate local anesthesia, intravenous sedation, and maternal antibiotics are provided, a 3.8 mm trocar will be inserted under ultrasound guidance through a 2-3 mm skin incision into the amniotic cavity of the normally grown twin. The communicating vessels will be identified endoscopically and photocoagulated with YAG laser energy. Patients will remain hospitalized for 24-48 hours. Follow-up ultrasounds will be scheduled every week for the first month to detect possible intrauterine fetal demise, and monthly thereafter. Delivery will be decided based on obstetrical indications.
For further reading, please see the link below:
